THe Science!

"Based on science, made for play."
Game Juice is the world's first and only cleaning solution specifically designed for video games.
HOW IT WORKS

All metals oxidize when exposed to the elements in the form of corrosion. This is a fact of nature. Moisture (blowing!) and external contaminants can catalyze the reactions. While DIY fixes such as isopropyl alcohol work fine as a general organic solvent, Game Juice® works best because it was formulated specifically with game cartridges in mind. The premium formula works to slow down the redox reaction and corrosion that occur with a stronger more dynamic solution. While there is no way to completely stop the many chemical reactions and corrosion effects from aging unless a chemical sealant is put on the cartridge (highly in-advised) - with regular use, Game Juice® can help mitigate these effects.G




We recommend cleaning stored games every 2-3 months to keep them happy! If cartridges are being played regularly, Game Juice® should be used during every session or at minimum every 2-3 times the game is played. X
After years of testing and development let’s get into the nitty gritty and explore some of the research and thought that went into creating our premium solution Game Juice® shall we?.....SCIENCE!
Components and Materials
What game cartridges and system connectors are made of:
Cartridges pins and system connectors
-
Copper Alloys
-
Nickel
-
Tin
-
Aluminum
-
Zinc Alloys
-
Iron Alloys
-
Gold plated alloys
Circuit boards and PCBs (Printed Circuit Boards)
-
Beryllium, cadmium, coltan, copper, gold, lead, lithium, nickel, silcon, silicon oxynitride, silver, tantalum, zinc, and more…(wow!)
Casing and housing polymers (plastics)
The most common include:
-
Polypropylene
-
Impact styrene
-
Acrylonitrile Butadiene Styrene (ABS)
-
and others...
These are made from crude oil.
CORROSION
"The worst enemy!"
CORROSION DEFINITION:
Corrosion is a chemical and/or electrochemical process usually involving metals in which X deterioration occurs through oxidation and exposure to the surrounding environment. The type of metal, environmental conditions, the presence of stresses/defects and the form of the metal determine the rate of deterioration.

THE CORROSIVE PROCESS
In essence, the corrosion of metals is an electron transfer reaction. An uncharged metal atom loses one or more electrons and becomes a charged metal ion:
M⇌M++electron
In an ionizing solvent the metal ion initially goes into solution but may then undergo a secondary reaction, combining with other ions present in the environment to form an insoluble molecular species such as rust or aluminum oxide. In high-temperature oxidation the metal ion becomes part of the lattice of the oxide formed.

Z
Most forms of corrosion result from electrochemical reactions. General corrosion occurs when most or all of the atoms on the same metal surface are oxidized, damaging the entire surface. Most metals are easily oxidized: they tend to lose electrons to oxygen (and other substances) in the air or in water. As oxygen is reduced (gains electrons), it forms an oxide with the metal.
The most commonly known form of this type of corrosion is hydrated iron(III) oxide Fe2O3·nH2O and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3) or RUST.



MAIN TYPES OF CORROSION
-
Galvanic corrosion Z
The most common and impactful form of corrosion. It occurs when two dissimilar (different) metals are in contact in the presence of an electrolyte. In a galvanic cell (bimetallic couple), the more active metal (anode) corrodes and the more noble metal (cathode) is protected. There are a number of factors that affect the galvanic corrosion including types of metals, relative size of anode, and environment (temperature, humidity, salinity, etc.
-
Pitting Corrosion
Occurs under certain conditions, which leads to accelerated corrosion in certain areas rather than uniform corrosion throughout the piece. Such conditions include low concentrations of oxygen or high concentrations of chlorides (anions) that interfere with the alloys ability to reform a passivating film. In the worst cases, most of the surface remains protected, but tiny fluctuations degrade the film in a few critical areas. Corrosion at these points is amplified and can cause pits.
-
Microbial corrosion
Commonly referred to as microbiologically influenced corrosion (MIC) is caused by microorganisms. It applies to both metallic and non-metallic materials with or without oxygen. When oxygen is absent, sulfate-reducing bacteria are active and produce hydrogen sulfide causing sulfide stress cracking. When oxygen is present, some bacteria may directly oxidize iron to iron oxides and hydroxides. Concentration cells can form in the deposits of corrosion products, leading to localized corrosion.
-
High-temperature corrosion
As the name suggests, this type of corrosion is the deterioration of a metal due to heating. This can occur when a metal is subjected to a hot atmosphere in the presence of oxygen, sulfur, or other compound capable of oxidizing the material.
-
Crevice corrosion
Occurs in confined spaces where access of fluid from the environment is limited such as gaps and contact areas between parts, under gaskets or seals, inside cracks and seams and spaces filled with deposits and contaminants.
Video Games and Copper


L
VIDEO GAMES AND COPPER CORROSION
Since copper, aluminum, tin and nickle are some of the most common metals found in game cartridge contacts and system connectors let’s look at some examples of corrosion in action.
Copper corrosion is the corrosion of materials made of copper or copper alloys. When exposed to the atmosphere, copper oxidizes, cause bright copper surfaces to tarnish. After a few years, this tarnish gradually changes to dark brown or black, and finally to green.

Copper corrodes at negligible rates in unpolluted air, water and deaerated non-oxidizing acids. However, it is susceptible to more rapid attack in oxidizing acids, oxidizing heavy-metal salts, sulfur, ammonia, and some sulfur and ammonia compounds

There are two types of copper corrosion:
Uniform - Identified by the presence of a relatively uniform layer of copper corrosion byproducts across the inner surface of a pipe wall. It is typically associated with elevated copper levels in certain areas of a structure composed of copper.
Non-uniform - Isolated development of corrosion cells across the surface or wall of a copper-based structure. Excessive pitting corrosion can lead to holes and gaps throughout the surface.
The most Famous Form of Copper Corrosion
Statue of Liberty: Changing Colors
The Statue of Liberty is a landmark every American recognizes. The Statue of Liberty is easily identified by its height, stance, and unique blue-green color. When this statue was first delivered from France in 1886, its appearance was not green. It was brown, the color of its copper “skin.” So how did the Statue of Liberty change colors? The change in appearance was a direct result of corrosion. The copper that is the primary component of the statue slowly underwent oxidation from the air. The oxidation-reduction reactions of copper metal in the environment occur in several steps. Copper metal is oxidized to copper(I) oxide (Cu2O), which is red, and then to copper(II) oxide, which is black.

Coal, which was often high in sulfur, was burned extensively in the early part of the last century. As a result, sulfur trioxide, carbon dioxide, and water all reacted with the CuO
These three compounds are responsible for the characteristic blue-green patina seen today. Fortunately, formation of the patina created a protective layer on the surface, preventing further corrosion of the copper skin.

Corrosion Prevention
The World Corrosion Organization estimates the global cost of corrosion to be about US$ 2.2 trillion annually, and that a large portion of this - as much as 25% - could be eliminated by applying simple, well-understood prevention techniques.
CALCULATING CORROSION
The Nernst equation is a mathematical description of ideal pH electrode behavior and correlating chemical energy as well as the electric potential of a galvanic cell or battery. It shows the relationship between the potential of a half cell or full cell at any point in time and the standard electrode potential, activity, temperature, reaction quotient of the species used and the underlying conditions.
When a redox reaction occurs in a galvanic cell, the concentration of the reactants decreases as they are consumed while the concentration of the products increase due to more product formation, and the cell potential decreases until equilibrium is reached and a zero cell potential is reached.
During the reaction, the Nernst equation can be used to determine the cell potential at any instant and conditions different from the standard state.
NERNST EQUATION GENERAL FORM

V
Where:
-
Ecell = cell potential under nonstandard conditions (V)
E0cell = cell potential under standard conditions
R = gas constant, which is 8.31 (volt-coulomb)/(mol-K)
T = temperature (kelvin), which is generally 298°K (77°F/25°C)
n = number of moles of electrons exchanged in the electrochemical reaction (mol)
F = Faraday's constant, 96500 coulombs/mol
Q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations
The equation can be rearranged to give ln Kc = nFE/RT where Kc is the equilibrium constant at the equilibrium state. The equilibrium potential is dependent on temperature and concentration of reaction partners.
The Nernst equation used in for:
-
Accurate determination of equilibrium constants
-
Determining voltage and concentration of a component of an electrochemical cell
-
Calculating the potential developed by a concentration cell (in corrosion)
-
Construction of Pourbaix diagram showing the equilibrium potential between a metal and its various oxidized species as a function of pH


Solvents
SOLVENT DEFINITION:
Solvent (from the Latin solvō, "loosen, untie, solve"). A substance, ordinarily a liquid, in which other materials dissolve to form a solution.
Their chief uses are as media for chemical syntheses, as industrial cleaners, in extractive processes, in pharmaceuticals, in inks, and in paints, varnishes, and lacquers.

MOLECULAR STRUCTURES OF SOLVENTS:
Ability of a substance to dissolve another substance is determined by compatibility of their molecular structures (like dissolves like).
Polarity and Proticity
Proticity is the characterization of a substance as protic (a hydrogen bond donor) or aprotic (incapable of donating a hydrogen bond).
Aprotic solvents lack a hydrogen atom completely or contains one with an insufficiently large δ+ so as to be unable to be a hydrogen bond donor.
Protic solvents have a hydrogen atom with a sufficiently large δ+ on the hydrogen atom, therefore possessing the ability to be a hydrogen bond donor.
Polarity refers to the electronegativity difference of the elements forming bond. A large difference creates a slightly positive(δ+) side and slightly negative side (δ-) or dipole moment. Molecules can be either polar or non-polar in reference to the bonds between elements.

Polar solvents (e.g., water) favour formation of ions; nonpolar ones (e.g., hydrocarbons) do not. Solvents may be predominantly acidic, predominantly basic, amphoteric (both), or aprotic (neither). Organic compounds used as solvents include aromatic compounds and other hydrocarbons, alcohols, esters, ethers, ketones, amines, and nitrated and halogenated hydrocarbons. A more detailed explanation of the types of molecular structures of the solvents are as follows:
Polar protic solvents
A polar protic molecule consists of a polar group (OH) and a non-polar tail. The structure may be represented by a R-OH. Polar protic solvents dissolve other substances with polar protic molecular structures. Polar protic solvents are miscible with water (hydrophilic).
Examples of polar protic solvents: water (H-OH), acetic acid (CH3CO-OH), methanol (CH3-OH), ethanol (CH3CH2-OH), n-propanol (CH3CH2CH2-OH), and n-butanol (CH3CH2CH2CH2-OH).

Dipolar aprotic solvents
Dipolar aprotic molecules possess a large bond dipole moment (a measure of polarity of a molecule chemical bond). They do not contain an OH group.
Examples of dipolar aprotic solvents: acetone ( (CH3)2C=O ), ethyl acetate (CH3CO2CH2CH3), dimethyl sulfoxide ( (CH3)2SO ), acetonitrile (CH3CN) and dimethylformamide ( (CH3)2NC(O)H ).
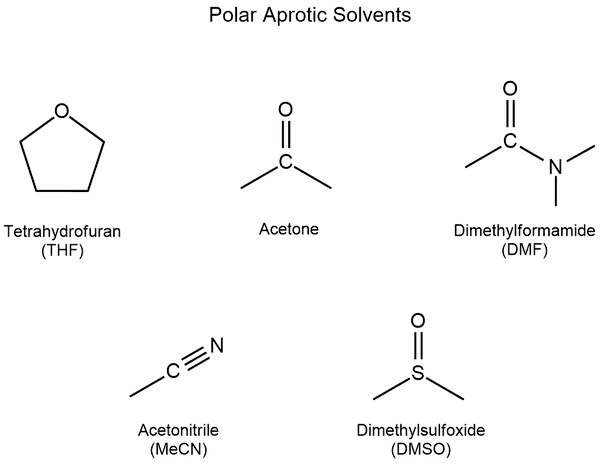
Non-polar solvents
Electric charge in the molecules of non-polar solvents is evenly distributed, therefore the molecules have low dielectric constant. Non-polar solvents are hydrophobic (immiscible with water). Non-polar solvents are liphophilic as they dissolve non-polar substances such as oils, fats and greases.
Examples of non-polar solvents: carbon tetrachloride (CCl4), benzene (C6H6), diethyl ether (CH3CH2OCH2CH3), hexane (CH3(CH2)4CH3) and methylene chloride (CH2Cl2).

INORGANIC SOLVENTS
Inorganic solvents are solvents the do not contain Carbon (C). The most popular inorganic solvents are water (H2O) and aqueous solutions containing special additives (surfacants, detergents, PH buffers, inhibitors).
Other inorganic solvents include liquid anhydrous Ammonia (NH3), concentrated sulfuric acid (H2SO4) and sulfuryl chloride fluoride (SO2ClF).

ORGANIC SOLVENTS
Oxygenated solvents I
Oxygenated solvent is an organic solvent, molecules of which contain oxygen. Oxygenated solvents are widely used in the paints, inks, pharmaceuticals, fragrance sectors, adhesives, cosmetics, detergents, food industries.
Examples of oxygenated solvents: alcohols, glycol ethers, methyl acetate, ethyl acetate, ketones, esters, and glycol ether/esters.
Hydrocarbon solvents
Molecules of hydrocarbon solvents consist only of hydrogen and carbon atoms.
-
Aliphatic solvents
Molecules of aliphatic solvents have a straight-chain structure.
Examples: Hexane, gasoline, kerosene are aliphatic solvents.
-
White spirits (mineral turpentine spirits)
White spirit is a mixture of aromatic and paraffinic hydrocarbons.
-
Pure aromatic solvents
Molecules of pure aromatic solvents have a benzene ring structure.
Examples of pure aromatic solvents are benzene, toluene and xylene.
Halogenated solvents
A Halogenated solvent is an organic solvent the molecules of which contain halogenic atoms: chlorine (Cl), fluorine (F) , bromine (Br) or iodine (I). According to the type of halogen present, halogenated solvents are classified into the following categories:
-
Chlorinated solvents
The common chlorinated solvents are trichlorethylene (ClCH-CCl2), perchlorethylene (tetrachlorethylene, Cl2C-CCl2), methylene chloride (CH2Cl2), carbon tetrachloride (CCl4)), cloroform (CHCl3), and 1,1,1-trichlorethane (methyl chloroform, CH3-CCl).
-
Fluorocarbon solvents
Examples of fluorocarbon solvents: dichlorofluoromethane (freon 21, CHCl2F), trichlorofluoromethane (freon 11, CCl3F), tetrafluoromethane (freon 14, CF4), difluorodichloromethane (freon 12, CHCl2F2), hydrochlorofluorocarbon (freon 22, HCFC)
-
Brominated solvents
Examples of brominated solvents: ethylene dibromide (1,2-dibromoethane, BrCH2-CH2Br), methylene chlorobromide (bromochloromethane, CH2BrCl), methyl bromine (bromomethane, CH2Br).
-
Iodinated solvents
Examples of iodinated solvents: n-butyl iodide (1-iodobutane, CH3CH2CH2CH2I), methyl iodide (iodomethane, CH3I), ethyl iodide (iodoethane, C2H5I), n-propyl iodide (1-iodopropane, CH3CH2CH2I).



References
Current Status and Future Prospects of Copper Oxide Heterojunction Solar Cells
Terence K. S. Wong 1,*, Siarhei Zhuk 1,2, Saeid Masudy-Panah 2 and Goutam K. Dalapati 2,*
https://www.mdpi.com/1996-1944/9/4/271/htm
American Galvanizers Association
Corrosionpedia
https://www.corrosionpedia.com/
The Electrochemical Society
The curated reference collection in Materials Science and Materials Engineering
Chemistry - Custom Ed. (Openstax)
Paul Flowers, PhD; Klaus Theopold, PhD; Richard Langley, PhD; William R. Robinson, PhD et al.
https://opentextbc.ca/chemistry/
UCLA - Chemistry/Organic Chemistry Department
https://www.chemistry.ucla.edu/
Understanding Biocorrosion, 2014 (ISBN 978-1-78242-125-2)
Turid Liengen, Damien Féron, Régine Basséguy and Iwona Beech
Electrochemistry, past and present. (American Chemical Society) ISBN 978-0-8412-1572-6
Orna, Mary Virginia; Stock, John (1989).
Classification of Solvents - Dr. Dmitri Kopeliovich
https://www.jtcroofing.co.uk/news/why-does-copper-turn-green/
